
| 상품 안내 및 환불, 교환, 배송문의 | |
| - 가게 전화번호 : | 1544-1900 |
| - 전화문의 시간 : |
오전 9시부터 오후 6시까지 (매주 월요일, 화요일, 수요일, 목요일, 금요일, 공휴일 제외) |
| - 가게 이메일 : | ink@kyobobook.co.kr |
| - 이용 택배회사 : | CJ대한통운 |
|
판매가게정보 |
|
| - 사업자명 : | (주)교보문고 |
| - 사업자등록번호 : | 102-81-11670 |
| - 통신판매업신고 : | 01-0653 |
|
- 현금영수증 : 발급가능 |
|
|
전화주문 및 결제문의 |
|
| - 꽃피는 아침마을 : | 1644-8422 |
|
가게와 직거래를 하시면 꽃송이 적립 및 각종 혜택에서 제외되고, 만일의 문제가 발생하는 경우에도 꽃마의 도움을 받으실 수 없습니다. 가게의 부당한 요구, 불공정 행위 등에 대해서도 꽃마로 직접 전화주세요. |
|

| 상세정보 | 구매후기 (0) | 상품Q&A (0) | 배송/교환/환불 안내 |
책소개열역학은 열과 일의 출입에 따라 나타나는 자연현상의 원리를 탐구하는 학문으로, 자연과학을 다루는 물리학, 화학은 물론 기계공학, 화학공학, 재료공학을 비롯한 공학 전반의 기초 학문일 정도로 매우 중요한 학문 분야입니다. 이에 따라 각 학과의 교육과정에서 필수 또는 핵심 교과목으로 지정하여 교육하고 있습니다. 수학/물리/화학을 공부해야 이들을 기반으로 이루어진 응용 과학 또는 공학 분야 교육과정을 이해할 수 있는 것처럼, 열역학도 과학/공학 분야에서 물리/화학 다음으로 기초를 이루는 매우 중요한 학문입니다. 그러함에도 불구하고 열역학은 처음 배우는 학생에게는 상당히 이해하기가 어려운 교과목으로 느껴지고 있습니다.
본 교재는 재료공학을 전공하는 학생들이 어려워하는 열역학을 최대한 이해하기 쉽게 그리고 내용이 체계적으로 연결될 수 있도록 구성함으로써, 처음 배우는 학생이 큰 어려움을 겪지 않고 열역학이라는 커다란 산을 오를 수 있도록 도와줄 목적으로 만들어졌습니다. 이를 위해 재료를 구성하고 있는 물질들의 안정성, 상태 변화, 그리고 화학 반응을 지배하는 근본 원리 등 핵심 개념을 체계적으로 설명하였습니다. 특히 엔트로피의 물리적 의미를 탐구해 봄으로써 자연현상이 어떤 원리로 일어나는지를 이해할 수 있게 합니다. 아울러 자유에너지 개념을 도입하여 특정 조건에서 재료가 어떠한 거동을 하는지를 예측할 수 있게 해주는 원리를 배움으로써, 기체의 거동, 용액의 형성, 상변태, 화학 반응 등 재료에서 관찰되는 다양한 현상을 정량적으로 해석하고 예측할 수 있는 능력을 배양합니다.
또한, 이 책은 이상기체·실제기체 모델, 이상용액·정규용액 모델 등 이론적 도구를 소개하고, 이를 화학반응의 평형(Ellingham 도표), 상평형(상태도), 전기화학적 평형(Nernst 방정식, Pourbaix 도표)과 같은 실제 문제에 어떻게 적용하는지 단계적으로 설명합니다. 이를 통해 열역학이 단순한 이론을 넘어 재료 설계, 개발, 공정 제어의 확고한 과학적 기반임을 분명히 알려주고 있습니다. 독자의 학습을 돕기 위해 각 장은 개념 설명과 물리적 해석을 우선하고, 필요한 수식은 맥락과 목적을 분명히 한 뒤 도입합니다. 핵심 정리, 예제, 요약, 개념 점검 문제를 통해 스스로 점검할 수 있도록 구성했습니다.
상세이미지 목차I. 서론
II. 열역학 제 1, 2, 3 법칙
III. 상태, 상태변수 그리고 프로세스
IV. 기체의 거동
IV-1 Boyle-Charles Gas Law
IV-2 van der Waals Equation
IV-3 Internal Energies of Ideal Gas and Real Gas
V. 상태 변화 과정과 열용량
V-1 Processes of Ideal Gas
i) 등체적과정 (Constant Volume Process)
ii) 등압과정 (Constant Pressure Process)
iii) 단열과정 (Adiabatic Process)
iv) 등온과정 (Isothermal Process)
V-2 Heat Capacity
V-3 Enthalpy, H
V-4 Temperature Change during an Adiabatic Process
VI. 엔트로피의 통계역학적 해석
VI-1 Definition of Entropy S
VI-2 Statistical Interpretation of Entropy
VI-3 Thermal Entropy and Configurational Entropy
VI-4 Boltzmann Distribution Function
VI-5 Proof of lnΩ being proportional to Clausius Entropy S
VII. 가역과정과 비가역과정
VII-1 Irreversible Process
VII-2 Entropy of Irreversible Processes
VIII. 보조함수
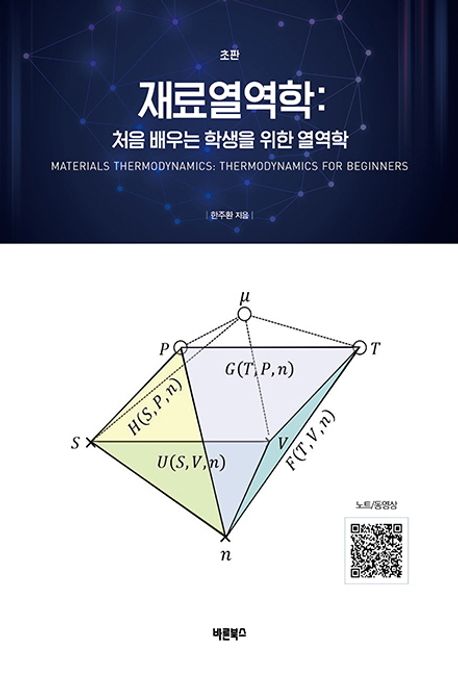
VIII-1 Geometric Relations
VIII-2 Auxiliary Functions
(1) Enthalpy
(2) Helmholtz Free Energy
(3) Gibbs Free Energy
VIII-3 Differentials of Auxiliary Functions
VIII-4 Maxwell Relations
VIII-5 Applications of Maxwell Relation using Experimentally determined Parameters
IX. 엔탈피와 엔트로피 함수 그리고 Gibbs 자유에너지
IX-1 Enthalpy as a function of Temperature under a constant Pressure
IX-2 Entropy as a function of Temperature under a constant Pressure
IX-3 Gibbs Free Energy as a function of Temperature under a constant Pressure
IX-4 Phase Diagram determined from Gibbs Free Energy Change with Temperature and Pressure
IX-5 Clapeyron Equation
IX-6 Clausius-Clapeyron Equation
X. 기체의 거동
X-1 Temperature Variation by Heat Transfer under constant Pressure
X-2 Pressure Variation by Work Transfer under constant Temperature
X-3 van der Waals Gas Model describing Real Gas Behavior
XI. 혼합 기체의 거동
XI-1 Gibbs Free Energy Variation with Pressure under constant Temperature
XI-2 Mixture of Ideal Gases
XI-3 Partial Molar Quantities and their Physical Meanings
XI-4 Chemical Potential as a Partial Molar Quantities
XI-5 Equilibrium Vapor Pressure of a Component in Condensed Solutions
XI-6 Activity and Activity Coefficient
XI-7 Raoult's Law and Henry's Law
XI-8 Chemical Potential and Activity
XII. 화학반응 평형
XII-1 Reactions involving Gases
XII-2 Effect of Temperature on Chemical Reaction Equilibrium
XII-3 Reference Pressure and Effect of Total Pressure on Chemical Reactions
XII-4 Reactions involving Pure Condensed Phases and a Gaseous Phase
(1) CO/CO2 비율 조절을 이용한 산소분압 조절
(2) H2/H2O 비율 조절을 이용한 산소분압 조절
XII-5 Reactions involving Condensed Solution Phases and a Gaseous Phase
XIII. 상태도
XIII-1 Free Energy Change as a Solute dissolving into a Matrix
XIII-2 Gibbs Free Energy of a Binary System
XIII-3 Ideal Solution
XIII-4 Development of Phase Diagrams
XIII-5 Development of Eutectic Phase Diagrams
XIII-6 Development of Other Phase Diagrams
XIV. 용액모델
XIV-1 Non-ideal Mixing
XIV-2 Regular Solution Model
XV. 전기화학
XV-1 Ionization Energy of an Element
XV-2 Equilibrium in Two Phase Systems involving an Electrolyte
XV-3 Equilibrium in an Electrochemical Cell
XV-4 Standard Hydrogen Electrode
XV-5 Pourbaix Diagram
(1) The Stability of Water
(2) Pourbaix Diagram for Copper
출판사 서평본 도서는 『재료열역학: 처음 배우는 학생을 위한 열역학』이라는 제목으로, 재료열역학을 처음 접하는 학생들을 위해 한주환 저자가 집필했습니다. 열역학은 기계공학과 화학공학을 포함한 공학 전반의 필수적인 기초 학문이며, 본 교재는 이러한 열역학적 원리를 재료 시스템에 특화하여 적용함으로써, 주어진 조건에서 재료 내부에서 일어나는 변화와 그 원리를 이해하는 데 목적을 둡니다.
[교재의 핵심 내용 및 구성]
* 열역학의 근본 원리 체계적 설명: 본 교재는 에너지 보존(제1법칙), 자발적 과정에서의 엔트로피 증가(제2법칙), 엔트로피의 절대 기준(제3법칙)의 세 가지 경험적 법칙을 기반으로 핵심 개념을 체계적으로 설명합니다.
* Gibbs 자유에너지 중심의 예측: 특히 자발적 변화의 방향과 평형을 가늠하는 핵심 상태함수인 Gibbs 자유에너지 G를 통해 기체의 거동, 용액의 형성, 상변태, 화학 반응 등 다양한 재료 현상을 정량적으로 해석하고 예측할 수 있게 합니다.
* 응용 분야 및 모델 소개: 이상기체/실제기체 모델, 이상용액/정규용액 모델 등 이론적 도구를 소개하고, 이를 화학반응 평형(Ellingham 도표), 상평형(상태도), 전기화학적 평형(Nernst 방정식, Pourbaix 도표)과 같은 실제 문제에 단계적으로 적용하는 방법을 설명합니다.
* 학습 편의를 위한 구조: 독자의 학습을 돕기 위해 각 장은 개념 설명과 물리적 해석을 우선하며, 필요한 수식은 맥락과 목적을 분명히 한 뒤 도입했습니다. 또한, 핵심 정리, 예제, 요약, 그리고 개념 점검 문제를 통해 스스로 학습 내용을 점검할 수 있도록 구성되어 있습니다.
* 추가 학습 자료 제공: 강의 노트와 영상 자료는 ‘https://ceramic.yu.ac.kr’에서 제공될 예정입니다.
본 교재는 열역학이 단순한 이론을 넘어 재료 설계, 개발, 공정 제어의 확고한 과학적 기반임을 분명히 하며, 재료의 안정성, 상변화, 화학 반응을 지배하는 근본 원리를 중심으로 깊이 있는 이해를 돕습니다. |
| 교환 및 환불 가능 |
상품에 문제가 있을 경우 |
1) 상품이 표시/광고된 내용과 다르거나 불량(부패, 변질, 파손, 표기오류, 이물혼입, 중량미달)이 발생한 경우 - 신선식품, 냉장식품, 냉동식품 : 수령일 다음날까지 신청 - 기타 상품 : 수령일로부터 30일 이내, 그 사실을 안 날 또는 알 수 있었던 날로부터 30일 이내 신청 2) 교환 및 환불신청 시 판매자는 상품의 상태를 확인할 수 있는 사진을 요청할 수 있으며 상품의 문제 정도에 따라 재배송, 일부환불, 전체환불이 진행됩니다. 반품에 따른 비용은 판매자 부담이며 환불은 반품도착일로부터 영업일 기준 3일 이내에 완료됩니다. |
|
단순변심 및 주문착오의 경우 |
1) 신선식품, 냉장식품, 냉동식품 재판매가 어려운 상품의 특성상, 교환 및 환불이 어렵습니다. 2) 화장품 피부 트러블 발생 시 전문의 진단서 및 소견서를 제출하시면 환불 가능합니다. 이 경우 제반비용은 소비자 부담이며, 배송비는 판매자가 부담합니다. 해당 화장품과 피부 트러블과의 상당한 인과관계가 인정되는 경우 또는 질환치료 목적의 경우에는 진단서 발급비용을 판매자가 부담합니다. 3) 기타 상품 수령일로부터 7일 이내 신청, 왕복배송비는 소비자 부담 4) 모니터 해상도의 차이로 색상이나 이미지가 다른 경우 단순변심에 의한 교환 및 환불이 제한될 수 있습니다. |
|
| 교환 및 환불 불가 |
1) 신청기한이 지난 경우 2) 소비자의 과실로 인해 상품 및 구성품의 전체 또는 일부가 없어지거나 훼손, 오염되었을 경우 3) 개봉하여 이미 섭취하였거나 사용(착용 및 설치 포함)해 상품 및 구성품의 가치가 손상된 경우 4) 시간이 경과하여 상품의 가치가 현저히 감소한 경우 5) 상세정보 또는 사용설명서에 안내된 주의사항 및 보관방법을 지키지 않은 경우 6) 사전예약 또는 주문제작으로 통해 소비자의 주문에 따라 개별적으로 생산되는 상품이 이미 제작진행된 경우 7) 복제가 가능한 상품 등의 포장을 훼손한 경우 8) 맛, 향, 색 등 단순 기호차이에 의한 경우 |
|